Web Resources for Xenopus laevis
Introduction
Two species of Xenopus are commonly used by scientists: Xenopus laevis and Xenopus Tropicalis. They are amphibians and both species originate from Africa. To see the differences between, please, visit the following link: http://xenbase.org/anatomy/intro.do
Fig.1 Xenopus leavis (on the left) and tropicalis (on the right)
Xenopus laevis lives in warm grassland ponds as well as in streams in arid and semi-arid regions and can tolerate wide variation of water pH. It has interesting morphology as it lacks tongue and visible ear and smooth slippery skin which is multicolored on its back. For more information:
http://animaldiversity.ummz.umich.edu/accounts/Xenopus_laevis/#habitat
http://en.wikipedia.org/wiki/African_clawed_frog
http://www.xlaevis.com/frogs.html
http://php.med.unsw.edu.au/embryology/index.php?title=Frog_Development#Frog_Life_Cycle
http://www.youtube.com/watch?v=r4MPSfxMVhU
Xenopus laevis is best known of all Xenpous species and is commonly studied as a model organism for developmental biology, cell biology, toxicology and neuroscience as the ease of maintenance, induced spawning and robustness of the embryos.
Why using Xenopus laevis in research?
The answers can be found at:
http://www.xlaevis.com/why.html
http://www.labome.com/method/Xenopus-laevis-as-a-Model-System.html
For the role of Xenopus laevis in the past, please, visit : http://xenbase.org/anatomy/intro.do
Biology of Xenopus laevis
Mating
Xenopus laevis mating is most common in spring but can take place during almost any time of the year. Usually the male is around 20 % smaller than the female. For more information:
http://aquaticfrogs.tripod.com/id3.html
http://animaldiversity.ummz.umich.edu/accounts/Xenopus_laevis/#reproduction
http://www.xlaevis.com/sexing.html
There are two ways of producing Xenopus embryos :
Natural mating
- the adult male and female are injected with chorionic gonadotropin hormone
- put them together
- wait for the amplexus to happen-male grabs the female and fertilizes the eggs that female produce.
- Collect the fertilized embryos and eggs

Fig.2 Xenopus laevis mating.
The advantage of this method is the fact that many embryos are at different stages. It is good for expression study. More information can be found:
http://www.xlaevis.com/natural.html
http://www.youtube.com/watch?v=0vSkVrh0UME
IVF (In vitro Fertilization)
- Injecting the male and female with gonadotropin hormone
- Harvest the testes from male
- Collect unfertilized eggs from female
- Fertilize the eggs with testis

Fig.3 Xenopus laevis eggs after IVF.
In this type of fertilization many synchronized eggs are present. This method is good for injection experiments. For more information visit the following sites:
http://www.xlaevis.com/invitro.html
http://www.xlaevis.com/testes.html
Embryonic development of Xenopus laevis is described: http://xenbase.org/anatomy/alldev.do
Large yolky eggs and large embryos are observed. The development is external which means that the embryos are suitable for experimental manipulation and observation. The development of the general body plan is subdivided into: cleavage, gastrulation and nerolation.
Cleavage – for further info, please, see:
http://www.uoguelph.ca/zoology/devobio/210labs/frogcleavage.htm
After the fertilization the zygote undergoes number of cell cleavage divisions (2-cell, 4-cell, 8-cell and 16 cell stages) and the process results in the formation of large cells called blastomeres. A cavity called blastocoel has formed in the centre of the animal hemisphere. This structure is called blastula.
Fig.4 Blastula
More scientific information in details can be read here:
http://www.vivo.colostate.edu/hbooks/pathphys/reprod/fert/cleavage.html
http://www.youtube.com/watch?feature=player_embedded&v=fUpc93T12bk
Gastrulation: some interesting facts and more interesting information is given here:
http://www.uoguelph.ca/zoology/devobio/210labs/gastrulation2.html
http://embryo.asu.edu/pages/gastrulation-xenopus
Gastrulation is the process characterized with the formation of the three germ layers-endoderm, mesoderm and ectoderm. The movements involved in Xenopus leavis gastrulation are Epiboly, Invagination, Convergent extension and Convergent thickening.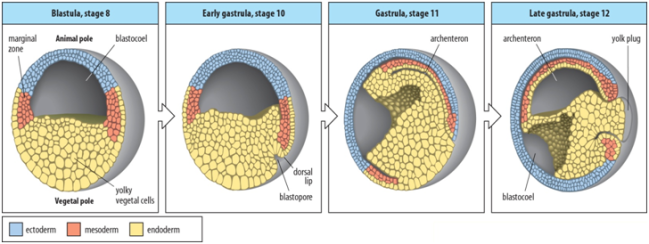
Fig.6 Gastrulation movements.
http://www.youtube.com/watch?feature=player_embedded&v=YlUsUH9G1Mo
Neurulation and later stages: useful info is represent: http://dev.biologists.org/content/23/2/427.full.pdf
The following stage of embryonic development is called neurula. During neurulation
the central nervous system is formed by the ectoderm on the dorsal side.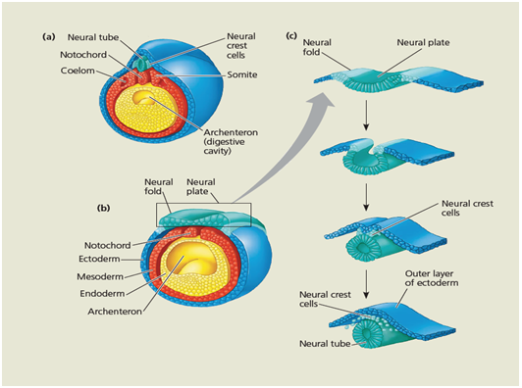
Fig. 7 Neurulation
http://www.youtube.com/watch?feature=player_embedded&v=D76B0K-D4rY
Tailbud Stage: detailed scientific inputs and facts are revealed here:http://dev.biologists.org/content/126/8/1611.full.pdf
By the tailbud stage all major body parts are in their final position.
Fig.8 Tailbud stage
Tadpole: the scientific researches of this stage are presented here:
http://aquaticfrogs.tripod.com/id4.html
Fig.9 Tadpole stage
Fate maps:
http://embryo.asu.edu/pages/fate-map
http://xenbase.org/anatomy/static/fate_maps/fates/16/16V2.2.jsp
Fig.10 Fate map
The results during research in years are provided: http://www.labome.com/method/Xenopus-laevis-as-a-Model-System.html
The biological activity and inhibition experiments are particularly easy in Xenopus because of the ease of injecting materials into embryos and the use of microsurgical isolation of explants or ultraviolet irradiation.To establish the function of any gene product in development it is necessary to know at least the expression pattern, the biological activity, and the effect of specific inhibition in vivo.
Experimental methods in Xenopus laevis
- in situ hybridisation – determines the expression pattern of a gene: http://www.genedetect.com/insitu.htm
 Fig.11 In situ hybridisation
Fig.11 In situ hybridisation
- Gain-of-function experiments
mRNA injections
The material to be injected will usually be an mRNA made in vitro. The RNA synthesis is performed using plasmids carrying promoters for RNA polymerase of bacteriophage such as Sp6. T3 or T7, and a poly (A) addition site to ensure in vivo addition of poly (A) to stabilize the mRNA. This mRNA can be injected into a whole fertilized egg, or into a specific blastomere during cleavage, which gives control over its location. The mRNA is translated in the egg and is likely to remain active throughout early development, but eventually will be degraded.
Example: Injection of dkk1 mRNA into Xenopus embryos causes development of big heads in those embryos. The reason is Dkk is Wnt inhibitor. For more information visit:
http://en.wikipedia.org/wiki/Wnt_signaling_pathway
Inducing activity of the introduced gene product
Doing this for transcription factor involves adding the hormone-binding domain from the glucocorticoid receptor to the protein of interest. This then causes it to bind to to the cytoplasmic protein hsp90, dextamethasone (lipid-soluble substance) is added. The dextamethasone can penetrate into the embryo and binds to the receptor, liberating it from hsp90 and allowing it to move to the nucleus and take part in gene regulation.
Fig.12 Animal cap assay
Isolated animal cap tissue differentiates into atypical ectoderm, but if treated with different concentrations of actin it develops into different cells and tissues.
Autoinduction animal cap assay
If the animal cap tissue has previously been injected with a component of mesoderminducing or neural-inducing systems and then is isolated, it will undergo the induction autonomously.
Example: If chordin mRNA(a BMP signaling pathway) is injected , the animal cap tissue differentiates as neural tissue.
Transgenesis in Xenopus : http://nar.oxfordjournals.org/content/28/4/e12.full.pdf+html
Very useful for studying events in late development as by this time the injected mRNA could have been degraded. There are several ways of making Xenopus transgenics. One of them is incorporating plasmid DNA into swollen sperm heads. They are then injected into unfertilized eggs and transgenic zygotes are created. It is usual to incorporate GFP coding sequence into the transgene. In this way the transgenic embryos are easily recognized. Xenopus transgenics are the most efficient in vertebrates.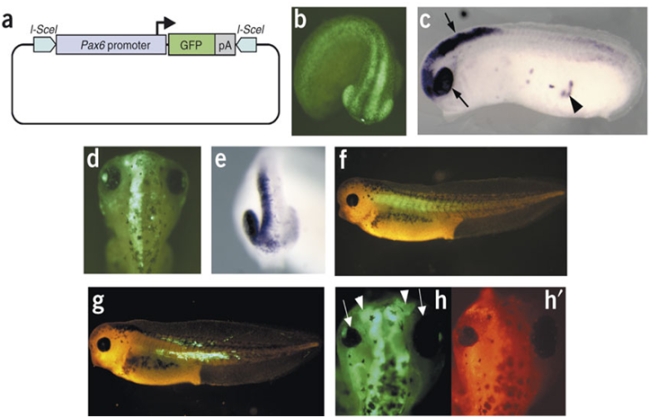
Fig.13 Transgenesis
- Loss-of-function experiments
Injection of antisense morpholino oligos into fertilized eggs: complete idea you would get by visiting this site:
http://mct.aacrjournals.org/content/1/5/347.full
Morpholinos hybridize to their complementary mRNA and block the translation process. Targeting the protein with an antibody is useful in order to show if the morpholino has been effective
Example: Injection of different control and Tcf/Lef Morpholinos into the right side of Xenopus embryo shows that of the four Tcf/Lef genes only Tcf and Tcf3 are required for mesoderm development.
Injection of antisense deoxyoligonucleotides
Antisense deoxyoligonucleotides are injected in the oocyte and they hybridize to the target mRNA. In this case the resulting hybrid is a target for the nuclease (RNAseH), which destroys the mRNA.
Domain-swapped transcription factors
The effector domain of the factor is replaced by strong activator or repressor. mRNA is made from each of these constructs and is injected into fertilized eggs. This gives information of the function of the studied transcription factor. This method can be used either for loss-of-function or gain-of-function experiments.
Experimental Embryology
Experimental Embryology involves Cut and Paste experiments.
- Isolating of ectoderm from the animal cap cells leads to formation of Epidermis.
- Combination of ectoderm from animal pole cells and dorsal mesoderm leads to the formation of neural tissue, so the dorsal mesoderm produce signaling molecules that induce formation of neural tissue in animal pole cells.
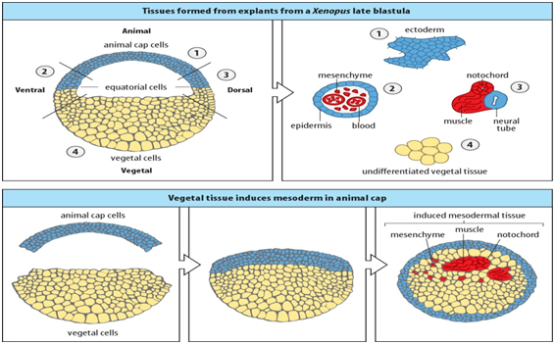
Fig. 14 Xenopus embryonic explants
Advantages and disadvantages of using Xenopus leavis in research:
|
Advantages |
Disadvantages |
|
vertebrate |
No genetics (under development) |
|
Ectopic gene expression possible in early embryos |
Difficult to create transgenic animals |
|
High accessibility of the embryo |
Levels of manipulation are difficult |
|
Excellent experimental embryology grafting induction preparations |
Not a mammal |
|
Injection of RNA into identifiable blastomeres |
Long generation time |
In case more info is needed , then click:
http://www.nih.gov/science/models/xenopus/advantages.html
http://mg1923.wix.com/african-claw-frog#!__advantages-and-disadvantages
References:
http://www.labome.com/method/Xenopus-laevis-as-a-Model-System.html
http://en.wikipedia.org/wiki/African_clawed_frog#Use_in_research
http://animaldiversity.ummz.umich.edu/accounts/Xenopus_laevis/#development
http://xenbase.org/anatomy/intro.do
http://www.xlaevis.com/testes.html
http://www.bio.davidson.edu/people/balom/stagingtable/Stage%2006/xenopus6.5.html
http://xenbase.org/anatomy/alldev.do
http://www.nih.gov/science/models/xenopus/advantages.html
http://mg1923.wix.com/african-claw-frog#!__advantages-and-disadvantages


